D-dimer
The biomarker of choice to aid the diagnosis of venous thromboembolism
-
 Fast results
Fast results
-
 High NPV
High NPV
-
 Reduced imaging*
Reduced imaging*
D-dimer testing in the emergency department
Point of care D-dimer testing* gives the possibility to rapidly test patients for venous thromboembolism (VTE), thus leading to a potential decrease in overcrowding in urgent care facilities [1].
Patients who present with signs and symptoms that may be caused by VTE require objective evaluation to exclude or confirm the diagnosis of a thrombus [2].
For patients at low or intermediate VTE risk, using D-dimer* as the initial test reduces the need for diagnostic imaging [3]. Imaging is associated with several disadvantages, e.g. radiation exposure, complications related to the administration of intravenous contrast and high healthcare costs [4].
*When used in combination with clinical assessment.
Immunoassay products and solutions
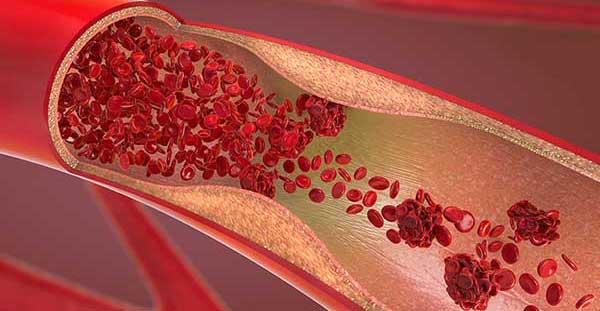

D-dimer is produced as the end product of fibrinolysis
The presence of D-dimer indicates that the in vivo coagulation system has been activated, forming a thrombus (blood clot) that initially stops a bleeding. D-dimer is the end product of the degradation that occurs in the healing process [5].
Venous thromboembolism
The most common presentation of VTE are deep vein thrombosis (DVT) and pulmonary embolism (PE). The causes can be multifactorial and both inherited or acquired [6].VTE is a condition that can have potentially serious and even life-threatening consequences [3].
Therefore, a fast decision have to be made on whether to exclude VTE by a negative D-dimer test or confirm by sending the patient for imaging.
How to use a D-dimer test
D-dimer* single test
An aid in ruling out the suspicion of DVT and PE [2]
D-dimer* + NT-proBNP
An aid in distinguishing PE diagnosis from heart failure in acutely dyspneic patients [7, 8]
D-dimer* + NT-proBNP and/or Troponin I
An aid in excluding PE and determining PE prognosis [3]
* When used in combination with clinical probability assessment.
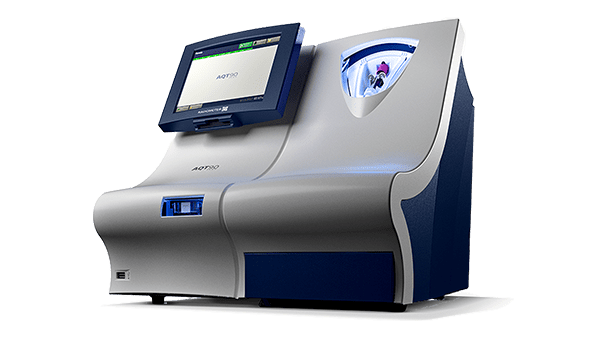
Testing D-dimer on the AQT90 FLEX analyzer
The AQT90 FLEX analyzer delivers lab-standard quantitative results on the D-dimer assay in less than 21 minutes, accelerating DVT and PE diagnosis in the ED to support rapid clinical decision making.
High negative predictive value (NPV) allows you to trust the D-dimer* results and help avoid sending ill patients home [5]. A high specificity means a lower number of false positive results. This minimizes unnecessary imaging on healthy persons, which is a benefit for the healthy person as well as for the healthcare system [5].
* In combination with clinical probability assessment.
The AQT90 FLEX immunoassay analyzer is a benchtop analyzer that brings rapid biomarker assessment capabilities right to the patient’s bedside.
Its closed tube system makes D-dimer testing easy: the operator simply inserts the sample tube into the tube holder in the sample port and the analyzer performs all assay steps automatically. There is no need for sample preparation.
All necessary reagents are included in the test cartridges, which remain stable onboard the analyzer for 31 days ensuring maximum availability and uptime.
Key benefits of D-dimer on the AQT90 FLEX analyzer
- No blood exposure: closed system
- No sample or assay preparation
- Specimen types: venous whole blood
- No presence of hook effect or carry-over
- Hemolysis, lipemia, icteria and biotin* show no interference with the assay
- Sample tubes: fit most 13 × 75 mm standard tubes
* No interference was observed with biotin up to 2.6 mg/L (2,600 ng/mL)
D-dimer assay specifics
> 50 years/whole blood 654 μg/L
References
2. CLSI. Quantitative D-dimer for the Exclusion of Venous Thromboembolic Disease; Approved Guideline. CLSI document H59-A. Wayne, PA: Clinical and Laboratory Standards Institute; 2011.
3. Konstantinides SV et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the EuropeanRespiratory Society (ERS). Eur Heart J. 2020; 41: 543-603.
4. Van der Hulle T, Dronkers CEA, Klok FA, Huisman MV. Recent developments in the diagnosis and treatment ofpulmonary embolism. JOIM 2015
5. Strandberg K. The clinical use of a D-dimer assay. acutecaretesting.org 2017
6. Lijfering WM, Rosendaal FR, Cannegieter. Risk factors for venous thrombosis - current understanding from an epidemiological point of view. Br J Haematol. 2010; 149, 6.
7. Stokes N, Dietz,B,and Liang J,Cardiopulmonary laboratory biomarkers in the evaluation of acute dyspnea, Open Access Emerg Med. 2016; 8: 35–45. page 10.
8. Lusky, Karen. One-two Punch in Evaluating PE Risk. Cap Today. Modified November 2006. http://www.captodayonline.com/Archives/feature_stories/1106pe_risk.html.
9.Sidelmann JJ, Gram J, Larsen A, Overgaard K, Jespersen J. Analytical and clinical validation of a new point-of-care testing system for determination of D-Dimer in human blood. Thrombosis Research 126 (2010) 524–530
Cookies are used on this website
Use of cookiesPlease enter a valid email
We will be sending an e-mail invitation to you shortly to sign in using Microsoft Azure AD.
It seems that your e-mail is not registered with us
Please click "Get started" in the e-mail to complete the registration process
Radiometer is using Microsoft AZURE Active Directory to authenticate users
Radiometer uses Azure AD to provide our customers and partners secure access to documents, resources, and other services on our customer portal.
If your organization is already using Azure AD you can use the same credentials to access Radiometer's customer portal.
Key benefits
- Allow the use of existing Active Directory credentials
- Single-sign on experience
- Use same credentials to access future services
Request access
You will receive an invitation to access our services via e-mail when your request has been approved.
When you accept the invitation, and your organization is already using AZURE AD, you can use the same credentials to access Radiometer's customer portal. Otherwise, a one-time password will be sent via e-mail to sign in.