
VET90 blood gas analyzer
A veterinary solution supporting you to deliver quality care to critically ill companion animals
-
 Quality care
Quality care
-
 Stay confident
Stay confident
-
 Patient focus
Patient focus
Every drop matters
Ideal for use in Emergency and Critical Care (ECC) facilities, the VET90 offers up to 18 key parameters, helping you make confident clinical decisions that can help get companion animals back on their paws, fast.
The VET90 blood gas analyzer - because every drop matters.
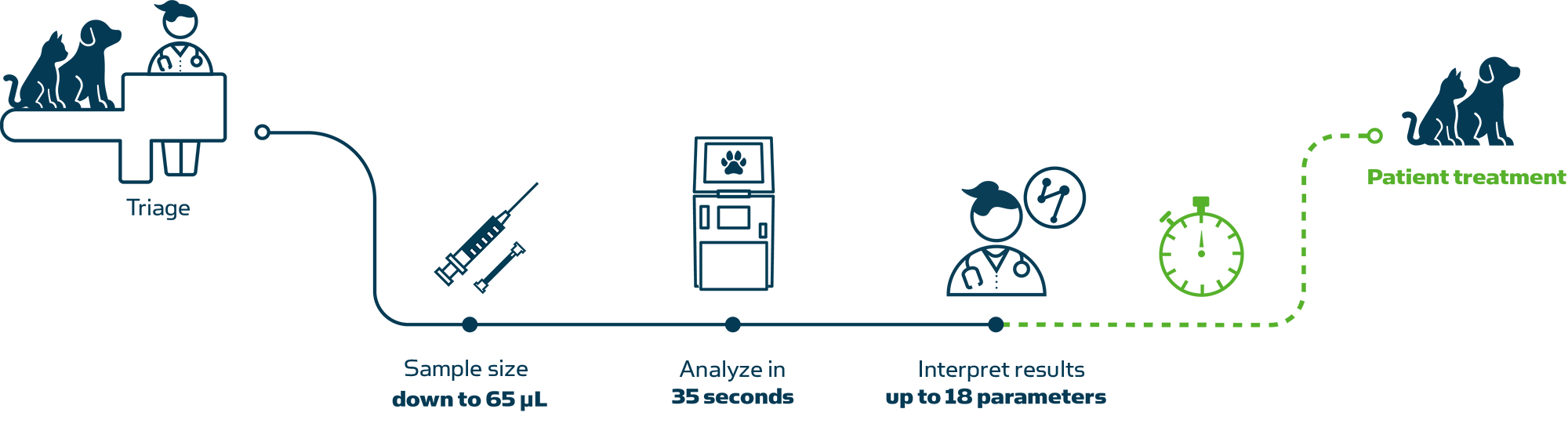
Analyze samples in three easy steps
Potential of hydrogen
The degree of acidity or alkalinity of any liquid (including blood) is a function of its hydrogen ion concentration [H+], and pH is simply a way of expressing hydrogen ion activity. The relationship between pH and hydrogen ion concentration is described thus:
pH = -log aH+
where aH+ is hydrogen ion activity.
Low pH is associated with acidosis and high pH with alkalosis [1].
1. CLSI. Blood gas and pH analysis and related measurements; Approved Guidelines. CLSI document CA46-A2, 29, 8. Clinical and Laboratory Standards Institute, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898 USA, 2009.
Partial pressure of carbon dioxide
Carbon dioxide (CO2) is an acidic gas; the amount of CO2 in blood is largely controlled by the rate and depth of breathing or ventilation. pCO2 is the partial pressure of CO2 in blood. It is a measure of the pressure exerted by that small portion (~5 %) of total CO2 that remains in the gaseous state, dissolved in the blood plasma. pCO2 is the respiratory component of acid-base balance and reflects the adequacy of pulmonary ventilation. The severity of ventilator failure as well as the chronicity can be judged by the accompanying changes in acid-base status [1].
1. Higgins C. Parameters that reflect the carbon dioxide content of blood. www.acutecaretesting.org Oct 2008.
Partial pressure of oxygen
The amount of oxygen in blood is controlled by many variables, e.g. ventilation/perfusion. pO2 is the partial pressure of oxygen in a gas phase in equilibrium with the blood. pO2 only reflects a small fraction (1 – 2 %) of total oxygen in blood that is dissolved in blood plasma [1]. The remaining 98 – 99 % of oxygen present in blood is bound to the hemoglobin in the erythrocytes. pO2 primarily reflects the oxygen uptake in the lungs.
1. Wettstein R, Wilkins R. Interpretation of blood gases. In: Clinical assessment in respiratory care, 6th ed. St. Louis: Mosby, 2010.
Glucose
Glucose, the most abundant carbohydrate in human metabolism, serves as the major intracellular energy source (see lactate). Glucose is derived principally from dietary carbohydrate, but it is also produced – primarily in the liver and kidneys – via the anabolic process of gluconeogenesis, and from the breakdown of glycogen (glycogenolysis). This endogenously produced glucose helps keep blood glucose concentration within normal limits, when dietary-derived glucose is not available, e.g. between meals or during periods of starvation.
Lactate
Lactate, the anion that results from dissociation of lactic acid, is an intracellular metabolite of glucose. It is produced by skeletal muscle cells, red blood cells (erythrocytes), the brain, and other tissues during anaerobic energy production (glycolysis). Lactate is formed in the intracellular fluid from pyruvate; the reaction is catalyzed by the enzyme lactate dehydrogenase (LDH) [1].
1. Robergs RA, Ghiasvand F, Parker D. Biochemistry of exercise-induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol 2004; 287: R502-16.
Creatinine
Creatinine is an endogenous waste product of muscle metabolism, derived from creatine, a molecule of major importance for energy production within muscle cells. Creatinine is removed from the body in urine and its concentration in blood reflects glomerular filtration and thereby kidney function.
Urea
Urea (molecular formula CO(NH2)2) is the principal nitrogenous waste product of protein catabolism, which is eliminated from the body in urine. It is the most abundant organic component of urine. Urea is transported in blood from the liver to the kidneys, where it is filtered from the blood and excreted in the urine. Renal failure is associated with the reduced excretion of urea in urine, and a consequent rise in blood (plasma/serum) urea concentration.
Calsium
The calcium ion (Ca2+) is one of the most prevalent cations in the body, where approximately 1 % is present in the extracellular fluid of blood. Ca2+ plays a vital role for bone mineralization and many cellular processes, e.g. contractility of the heart and the skeletal musculature, neuromuscular transmission, hormone secretion and action in various enzymatic reactions such as, e.g. blood coagulation.
Chloride
Chloride (Cl-) is the major anion in the extracellular fluid and one of the most important anions in blood. The main function of Cl- is to maintain osmotic pressure, fluid balance, muscular activity, ionic neutrality in plasma, and help elucidate the cause of acid-base disturbances.
Potassium
Potassium (K+) is the major cation in the intracellular fluid, where it has a 25 - 37-fold higher concentration (∼150 mmol/L in tissue cells, ∼105 mmol/L in erythrocytes) than in the extracellular fluid (∼4 mmol/L) [1, 2]. K+ has several vital functions in the body, e.g. regulation of neuromuscular excitability, regulation of heart rhythm, regulation of intracellular and extracellular volume and acid-base status.
1. Burtis CA, Ashwood ER, Bruns DE. Tietz textbook of clinical chemistry and molecular diagnostics. 5th ed. St. Louis: Saunders Elsevier, 2012. Engquist A. Fluids/Electrolytes/Nutrition. 1st ed. Copenhagen: Munksgaard, 1985.
2. Engquist A. Fluids/Electrolytes/Nutrition. 1st ed. Copenhagen: Munksgaard, 1985.
Sodium
Sodium (Na+) is the dominant cation in the extracellular fluid, where it has a 14-fold higher concentration (∼140 mmol/L) than in the intracellular fluid (∼10 mmol/L). Na+ is a major contributor of the osmolality of the extracellular fluid and its main function is largely in controlling and regulating water balance, and maintaining blood pressure. Na+ is also important for transmitting nerve impulses and activating muscle concretion.
Carboxyhemoglobin
FMetHb is the fraction of total hemoglobin (ctHb) that is present as methemoglobin (MetHb). By convention the fraction is expressed as a percentage (%).
In the range of 0 – 60 % COHb in arterial (COHb(a)) and venous blood (COHb(v)) is similar, i.e. either venous or arterial blood may be analyzed [1]. In most medical texts FCOHb(a) is referred to as simply COHb.
1. Lopez DM, Weingarten-Arams JS, Singer LP, Conway EE Jr. Relationship between arterial, mixed venous and internal jugular carboxyhemoglobin concentrations at low, medium and high concentrations in a piglet model of carbon monoxide toxicity. Crit Care Med 2000; 28: 1998-2001.
Bilirubin
Bilirubin is the yellow breakdown product of the degradation of the heme group of hemoglobin. It is transported in blood from its site of production – the reticuloendothelial system – to the liver, where it is biotransformed before excretion in bile. Jaundice, the pathological yellow discoloration of skin, is due to abnormal accumulation of bilirubin in the tissues, and is always associated with elevated blood concentration of bilirubin (hyperbilirubinemia).
Total hemoglobin
The concentration of total hemoglobin (ctHb) in blood includes oxyhemoglobin (cO2Hb), deoxyhemoglobin (cHHb), as well as the dysfunctional hemoglobin species that are incapable of binding oxygen:
carboxyhemoglobin (cCOHb) (see COHb), methemoglobin (cMetHb) (see MetHb) and sulfhemoglobin (cSulfHb).
Thus:
ctHb = cO2Hb + cHHb + cCOHb + cMetHb + cSulfHb
The rare sulfHb is not included in the reported c tHb in most oximeters.
Fraction of deoxyhemoglobin
FHHb in total hemoglobin in blood.
Methemoglobin
FMetHb is the fraction of total hemoglobin (ctHb) that is present as methemoglobin (MetHb). By convention the fraction is expressed as a percentage (%) [1].
In most medical text boxes MetHb(a) is referred to as simply methemoglobin (MetHb).
1. CLSI. Blood gas and pH analysis and related measurements; Approved Guidelines. CLSI document CA46-A2, 29, 8. Clinical and Laboratory Standards Institute, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898 USA, 2009.
Oxygen saturation
Oxygen saturation (sO2) is the ratio of oxyhemoglobin concentration to concentration of functional hemoglobin (i.e. oxyhemoglobin (O2Hb) and deoxyhemoglobin (HHb) capable of carrying oxygen [1].
The sO2 reflects utilization of the currently available oxygen transport capacity.
In arterial blood 98 – 99 % of oxygen is transported in erythrocytes bound to hemoglobin. The remaining 1–2 % of the oxygen transported in blood is dissolved in the blood plasma – this is the portion reported as partial pressure of oxygen (pO2) [2].
1. CLSI. Blood gas and pH analysis and related measurements; Approved Guidelines. CLSI document CA46-A2, 29, 8. Clinical and Laboratory Standards Institute, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898 USA, 2009.
2. Higgins C. Parameters that reflect the carbon dioxide content of blood. www.acutecaretesting.org Oct 2008.
Fraction of oxyhemoglobin
FO2Hb in total hemoglobin in blood.
Key benefits
![]()
![]()
![]()

Stay confident:
Analyze a sample on demand with more than 23 hours of uptime each day.
Make confident clinical decisions with Automatic Quality Management (AQM). AQM performs five continuous checks (analysis, calibration, QC, system and clot) that automatically detect errors, perform corrective actions and document issue resolution.

Patient focus:
Spend more time with your patients and less time in front of the analyzer with results in just 35 seconds.
With no need for preanalytical pipetting, you can spend this time making diagnostic and treatment decisions that support patient health.
Add canine and feline reference ranges to help you make informed treatment decisions.
Analyze samples in three easy steps
Why work with Radiometer?
Cookies are used on this website
Use of cookiesPlease enter a valid email
We will be sending an e-mail invitation to you shortly to sign in using Microsoft Azure AD.
It seems that your e-mail is not registered with us
Please click "Get started" in the e-mail to complete the registration process
Radiometer is using Microsoft AZURE Active Directory to authenticate users
Radiometer uses Azure AD to provide our customers and partners secure access to documents, resources, and other services on our customer portal.
If your organization is already using Azure AD you can use the same credentials to access Radiometer's customer portal.
Key benefits
- Allow the use of existing Active Directory credentials
- Single-sign on experience
- Use same credentials to access future services
Request access
You will receive an invitation to access our services via e-mail when your request has been approved.
When you accept the invitation, and your organization is already using AZURE AD, you can use the same credentials to access Radiometer's customer portal. Otherwise, a one-time password will be sent via e-mail to sign in.


